PCR & Gelelectrophoresis
- Study and Documents the Bento Biotech 101 Protoco.
- Especially: 4. Introduction to PCR
- Choose a Primer, find out what the primer is doing
- Document the Reagents used in the experiment
- Carefully Document any changes (MasterMix?) that h
- Did you document how to pour a gel? What devices did you use?
- Did you run a gel? Document the steps.
- Why did you need to add at a ladder. Describe what it does and how it is used.
- How exactly is the DNA visualized in the gel?
- Show pictures of a successful gel run.
Bento Lab is an experimental device that can perform a series of operations (centrifugation, PCR, gel electrophoresis, and gel observation) in genetic experiments in a single unit and is easily portable.
▼ Presentation by Philipp Boeing, developer of the Bento lab.
https://vimeo.com/369098071
Date of experiment: July 12, 2022
I accidentally deleted the photos taken on this day, so the pictures on this page were provided by Shiho Hasegawa and Tou Takuzen.
Under Takuzen’s supervision, we decided to examine Lactose Intolerance and Bitterness Tasting.
First, we did DNA extraction.
We followed the Bento Lab procedure, but in order to increase the concentration of DNA, after centrifugation at 8 kG 90 sec, 500 µl 70% ethanol was added, and centrifuged again at 8 kG 60 sec.
After centrifugation, the tubes were dried to remove water.

When thoroughly dry, we prepared PCR solution.
Preparation of PCR solution
- Primer-Mix 1ml (1uM) We used 2 primer-mix: Lactose Intolerance and Bitterness Tasting.
- taq 25ul
- dna 5ul
- primer mix 5ul
- te-buffer 15ul
Put DNA Template → primer mix → te-buffer → taq in the PCR tube in this order.
Taq polymerase thawed and put in at the last minute! Put it away in the freezer as soon as you use it!
Always replace the micropipette tip every time when the tube already contains another reagent!


PCR
This time we used miniPCR.

▼Cycle
94℃/120s
–
Number of cycles → 35 times
94℃/30s
64℃/30s
72℃/30s
–
72℃/120s
Gel making
TBE(0.5x): 50ml
Agar: 1g
Heated in microwave for 80 seconds.
Do not close the lid of the container too tightly when heating.

Add Gelgreen 3ul.
Electrophoresis
The solidified gel was placed in a Bluegel, poured with TBE, and electrophoresed.


▼result (After 40 min.)
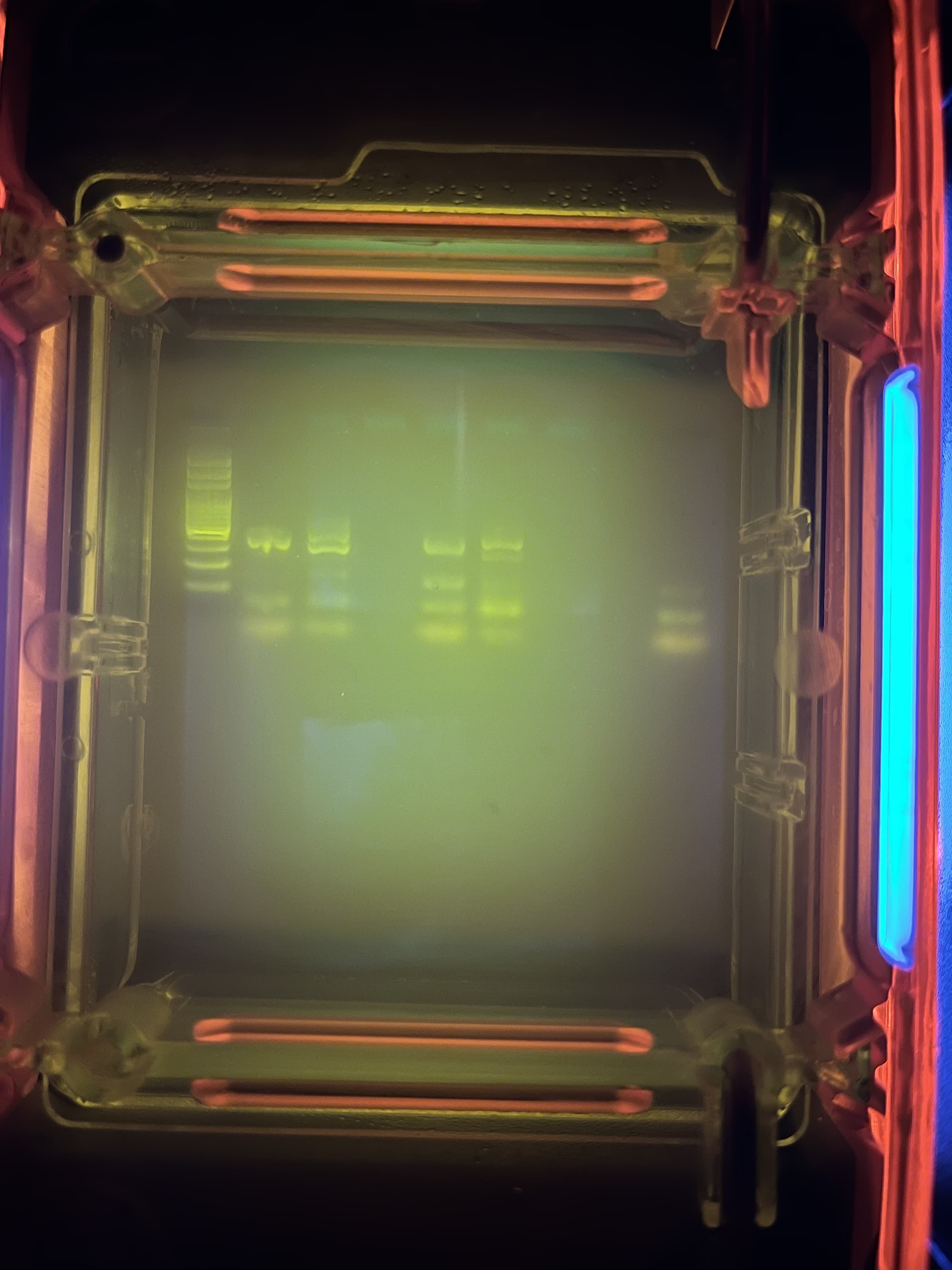
From left, Ladder, Bitterness Tasting (Hasegawa-san), Lactose Intolerance (Hasegawa-san), blank, Bitterness Tasting (Juppo), Lactose Intolerance (Juppo), blank, Only DNA (Hasegawa-san), Only TAC and Primer-Mix
The “ladder” is a sample containing multiple sizes of DNA and it functions as a ruler to measure the length of the DNA to be read.
Since DNA is negatively charged, it moves toward the positive electrode. The shorter (lighter) the DNA, the faster it moves. gel green-stained DNA emits fluorescence, indicating that a specific length of sequence has been amplified.
According to Bento Lab’s instructions, my sample corresponds to CC for Bitterness Tasting and CC or CT for Lactose Intolerance.
CC – Control (366 bp), C allele (151 bp)
This result shows a person who has both copies of the C allele, so homozygous dominant. This person should be able to taste bitterness.
https://bento.bio/protocol/biotechnology-101/bitter-taste/
Result CT – Control (440 bp), C allele (328 bp), T allele (166 bp)
This result shows a person with a copy of both the T allele and C allele, so someone who is heterozygous. The ability to digest lactose is dominant, so the person is likely to be able to digest milk as an adult.
CC – Control (440 bp), C allele (328 bp)
This result shows a person who has both copies of the C allele, so homozygous recessive. This person is likely to be lactose intolerant as an adult.
https://bento.bio/protocol/biotechnology-101/lactose-intolerance/
Why was it difficult to identify Lactose Intolerance?
It was difficult to determine whether a thin, wide band should be considered two bands or one band.
